Introduction
Gingipains, secreted by the bacterium Porphyromonas gingivalis, are a family of proteases with significant implications in oral health and disease. Gingipains are major virulence factors contributing to the pathogenicity of P. gingivalis in periodontal disease. All gingipains have a cysteine protease catalytic domain, characterized by a catalytic triad (cysteine, histidine, and asparagine) essential for proteolytic activity. Adhesin domains are found in RgpA and Kgp. These domains aid in binding to extracellular matrix components, enhancing the pathogenic potential of Porphyromonas gingivalis. Gingipains are categorized primarily into three types based on their substrate specificity and structural characteristics:
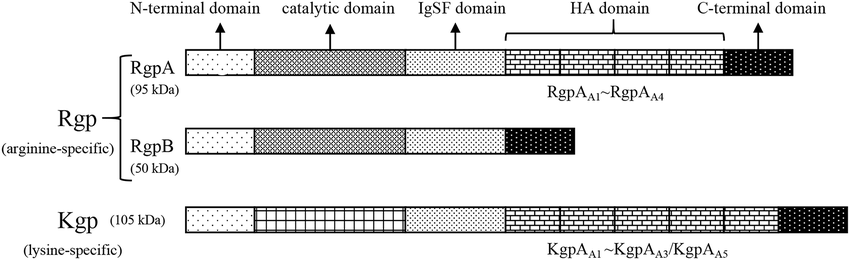
1. Arginine-specific Gingipains (Rgp):
These enzymes cleave peptide bonds at arginine residues and are divided into two types based on their structural and functional properties:
RgpA (Arg-gingipain A): It cleaves peptide bonds at the carboxyl side of arginine residues. It contains a catalytic domain and additional adhesin domains that facilitate binding to host tissues and proteins. It is involved in tissue invasion, immune evasion, and nutrient acquisition.
RgpB (Arg-gingipain B): Similar to RgpA, it cleaves peptide bonds at arginine residues.It consists of a catalytic domain without the additional adhesin domains found in RgpA, making it structurally more compact. It plays a key role in the degradation of host proteins and modulation of the host immune response.
2. Lysine-specific Gingipain (Kgp)
It cleaves peptide bonds at the carboxyl side of lysine residues. It comprises a catalytic domain and multiple adhesin domains similar to RgpA, facilitating binding and degradation of host tissues. It critical for tissue destruction, immune modulation, and nutrient acquisition.
How gingipains influence different types of immune cells
Neutrophils:
Chemotaxis and Activation: Gingipains can degrade chemotactic factors, such as interleukin-8 (IL-8), reducing neutrophil recruitment to the site of infection. They can also inactivate key receptors on neutrophils, impairing their ability to respond to chemotactic signals.
Apoptosis: Gingipains can induce apoptosis in neutrophils, thereby reducing their numbers and diminishing the host’s immediate immune response.
Macrophages:
Cytokine Production: Gingipains can modulate cytokine production by macrophages. For example, they can enhance the production of pro-inflammatory cytokines like TNF-α and IL-1β, contributing to chronic inflammation. Conversely, they can degrade certain cytokines and chemokines, diminishing the inflammatory response.
Phagocytosis: Gingipains can inhibit the phagocytic activity of macrophages by degrading opsonins and complement proteins that facilitate the recognition and ingestion of pathogens.
Dendritic Cells:
Maturation and Function: Gingipains can affect the maturation and antigen-presenting capabilities of dendritic cells. By degrading cytokines involved in dendritic cell maturation (e.g., IL-12), gingipains can impair the ability of these cells to activate T cells effectively.
Antigen Presentation: Gingipains can degrade surface molecules on dendritic cells that are critical for antigen presentation, thereby impairing the initiation of adaptive immune responses.
T Cells:
Activation and Proliferation: Gingipains can cleave co-stimulatory molecules and cytokines required for T cell activation and proliferation. This can lead to reduced T cell responses and impaired adaptive immunity.
Cytokine Modulation: Gingipains can influence the cytokine milieu, promoting a skewed T cell response that may favor regulatory T cells (Tregs) or Th17 cells, thereby altering the balance between pro-inflammatory and anti-inflammatory responses.
B Cells:
Antibody Production: Gingipains can degrade immunoglobulins and components of the complement system that are important for B cell activation and antibody production, leading to impaired humoral immunity.
Apoptosis: Similar to their effect on neutrophils, gingipains can induce apoptosis in B cells, reducing the overall B cell population and further compromising the antibody-mediated immune response.
Natural Killer (NK) Cells:
Cytotoxic Activity: Gingipains can impair the cytotoxic activity of NK cells by degrading activating receptors and cytokines that are essential for NK cell function.
Chemotaxis: By degrading chemotactic factors, gingipains can reduce the recruitment of NK cells to the site of infection, limiting their ability to contribute to the immune response.
Inflammation and Gingipains
Gingipains degrade cytokines, leading to a reduction in the host’s inflammatory response. Gingipains directly degrade pro-inflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α). This downregulation of inflammation may seem paradoxical, but it allows P. gingivalis to persist within the host without eliciting an overt immune response. Research suggests that gingipains may contribute to citrullination processes, thereby influencing inflammatory conditions. Citrullination, also known as deimination, is a post-translational modification of proteins wherein arginine residues are converted into citrulline residues. This process can be catalyzed by enzymes known as peptidylarginine deiminases (PADs). Gingipains might be involved in citrullination in following ways:
Direct Interaction: Gingipains could potentially modify PADs or other proteins, leading to an increased rate of citrullination.
Immune System Modulation: Gingipains may modulate the immune system in a way that affects PAD activity or expression, leading to increased citrullination.
Inflammatory Response: The inflammation caused by gingipains can create an environment that enhances the activity of PADs, promoting citrullination.
Modulation of complement system
The complement system is a crucial part of the innate immune response, involving a series of proteins that enhance the ability of antibodies and phagocytic cells to clear pathogens. Gingipains can modulate the complement system in following ways,
Degradation of Complement Proteins:
C3 and C3b: Gingipains can degrade C3, a central component of the complement system, and its activated form, C3b. This prevents the formation of the C3 convertase, which is essential for the amplification of the complement cascade.
C5: Gingipains can also cleave C5, preventing the formation of C5a and C5b, which are critical for the chemotactic response and the formation of the membrane attack complex (MAC), respectively.
Inactivation of Complement Receptors:
CR1: Gingipains can cleave complement receptor 1 (CR1) on the surface of erythrocytes and phagocytes, impairing the clearance of immune complexes and opsonized pathogens.
Interference with Complement Regulation:
Factor H and Factor I: Gingipains can degrade regulatory proteins such as factor H and factor I. These proteins normally inhibit the complement cascade to prevent damage to host tissues. By degrading these regulators, gingipains can dysregulate the complement system, contributing to tissue damage and inflammation.
Direct Inhibition of Complement Activation:
Classical and Alternative Pathways: Gingipains can directly inhibit the activation of both the classical and alternative complement pathways, thereby reducing the overall effectiveness of the complement system in clearing infections.
Subversion of Complement-Mediated Phagocytosis:
Opsonization: By degrading complement components and receptors involved in opsonization, gingipains reduce the ability of phagocytes to recognize and engulf P. gingivalis, allowing the bacteria to evade the immune response.
Conclusion
Gingipains exhibit sophisticated mechanisms simultaneously promoting bacterial survival while influencing the host’s immune landscape. The above stated mechanisms explain how gingipains help P. gingivalis evade the immune system and promote chronic infection and inflammation. Understanding these interactions is crucial for developing strategies to manage periodontal disease and its systemic effects.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:

